

Say for example more than one oxide is possible. Also, it does not provide complete information about the oxides and their formations.It does not consider the kinetics of the reactions.And as a result, this curve will go downwards. 2C (s)+ O 2 (g) → 2CO (g): Here one mole of gas is giving you two moles of gas as products.So there will be no slope, it is completely horizontal. So here one molecule of gas is resulting in one molecule of gas. C(s) + O 2 (g) → CO 2 (g): Entropy of solids is negligible.There are cases when the entropy is not negative, and the slope will not be upwards. Hence as the temperature increases, the value of TΔS will also increase, and the slope of the reaction goes upwards Also since in the reaction (as seen above), we are going from the gaseous state to the solid state ΔS is also negative.
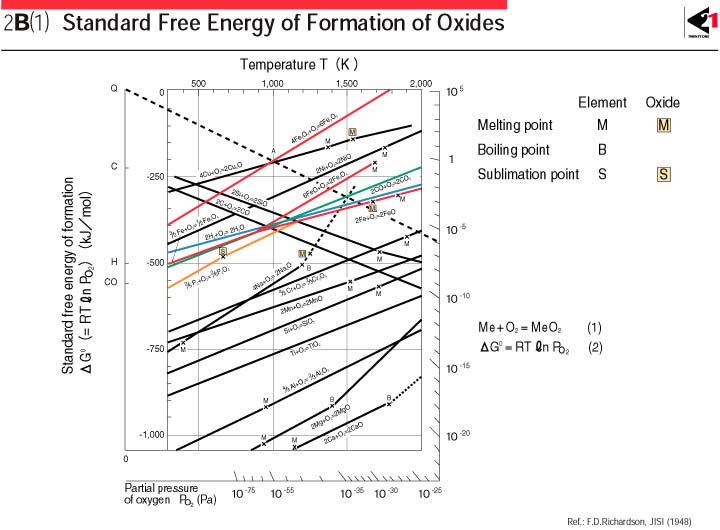
Now when reducing metal oxides the ΔH is almost always negative (exothermic) reaction. The reaction of metal with air can be generally represented as
#Ellingham diagrams free#
Another equation which relates the Gibbs Free Energy to the equilibrium constant is This changes very sharply when the state of the matter changes. ΔS is the Entropy or the randomness of molecules. So when the reaction is exothermic, it makes ΔG negative. Here a positive value will depict an endothermic reaction, while a negative value will be an exothermic reaction. We will now look at two equations to arrive at ΔG If this value of ΔG is negative then the reaction will occur spontaneously. In thermodynamics, whether a process will happen spontaneously or not will be determined by Gibbs Free Energy. The main thermodynamic concept we must concern ourselves with when it comes to metallurgy is Gibbs Free Energy. It also allows us to predict and measure these changes. Thermodynamics is the study of the energy transfer that occurs during chemical as well as physical changes. Thermodynamics is the branch of science that deals with a relationship between thermal energy i.e. And here is where the concept of thermodynamics exits. There is an overlap between the study of physics and chemistry, known as Physical Chemistry.


 0 kommentar(er)
0 kommentar(er)
